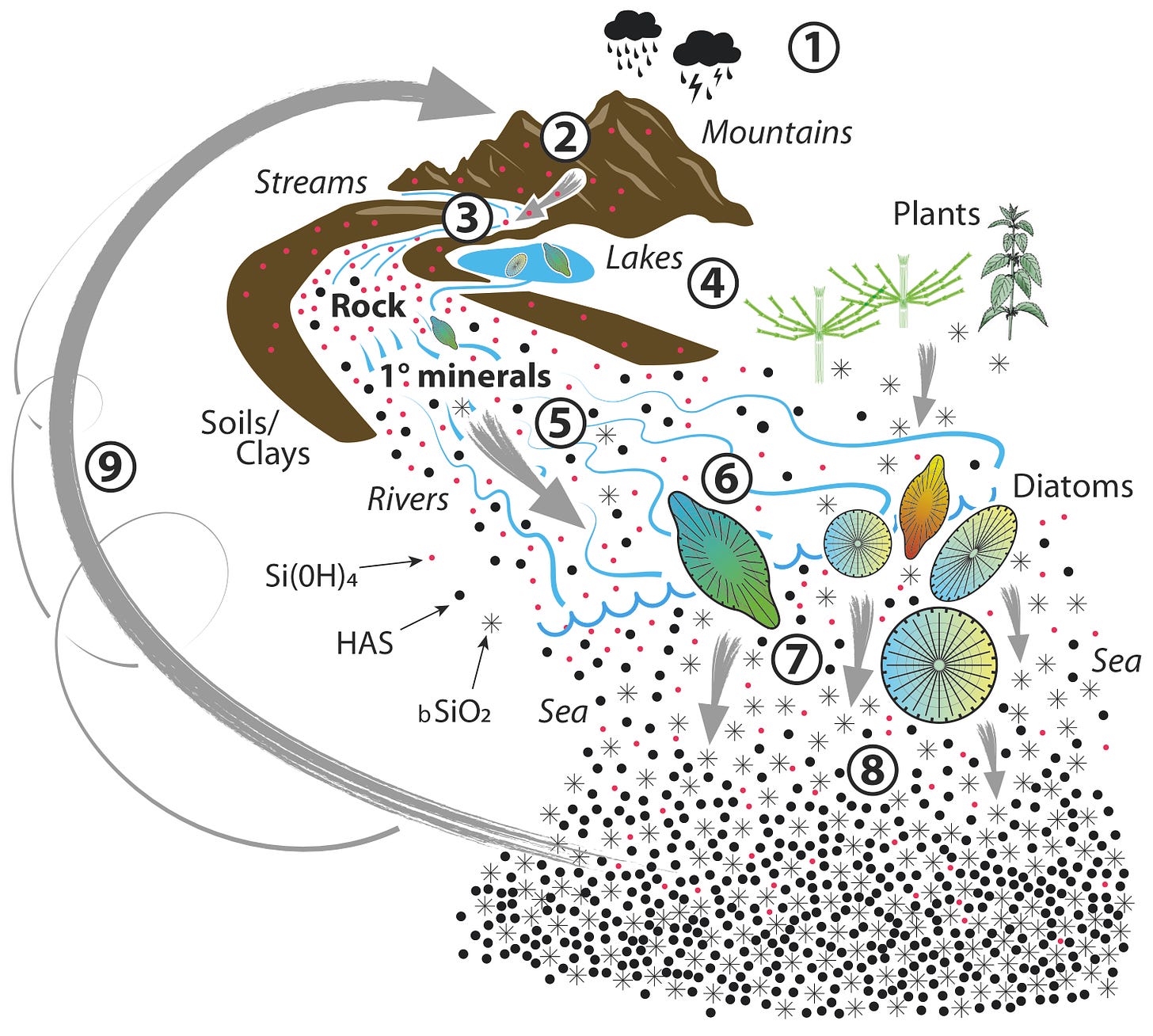
The story of silicon in life is essentially the story of silicic acid. The Earth’s crust is composed primarily of three elements, oxygen, silicon and aluminium. Perhaps the most prominent examples of the Earth’s crust are mountains. In their upper reaches the Earth’s crust is directly exposed to the environment, to the elements. Rainfall dissolves mountains and the first product of this dissolution is silicic acid. This is shown in the schematic below.
For a full description of this schematic, The Silicic Acid Cycle, please see my published paper. Silicic acid has been omnipresent in the living world since the beginning of time and yet silicon (silicic acid) is not by strict definition considered an essential element for life. Elements considered as essential have recognisable biochemistries such that their absence is inimical to an aspect of life. Silicon has never been shown to fulfil such a requirement. All living things can thrive in the absence of silicon. No identifiable silicon biochemistry means that there are no Si-C, Si-P, Si-N, Si-O-C…..et c. bonds required by any extant organism. But, in spite of all of this I would argue that life as we know it today would not exist if silicic acid had not always been omnipresent throughout biochemical evolution.
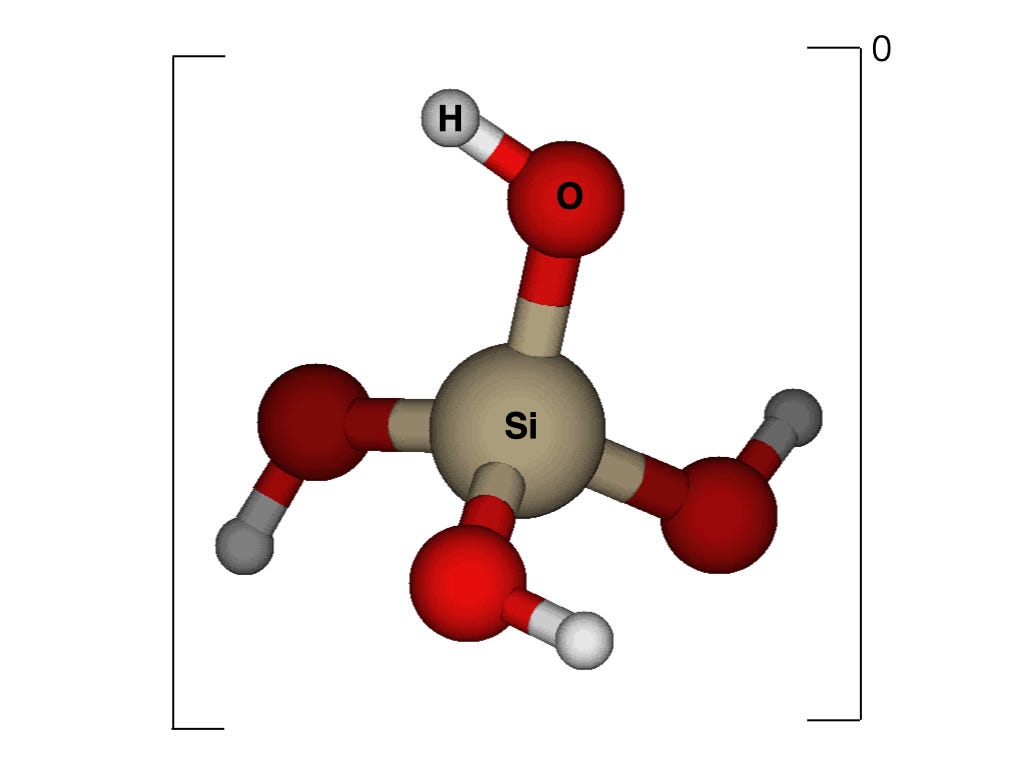
Let’s take a look at the structure of the super molecule silicic acid.
It is a silicon atom surrounded by four hydroxyl (OH) groups. It is called silicic acid because at pH approaching 10 (highly alkaline) one of the hydroxyl groups can release a proton, a hydrogen ion. Acids are so defined because of their propensity to donate protons. However, a pH approaching 10 is highly unusual in nature and so silicic acid is a neutral molecule to all intents and purposes.
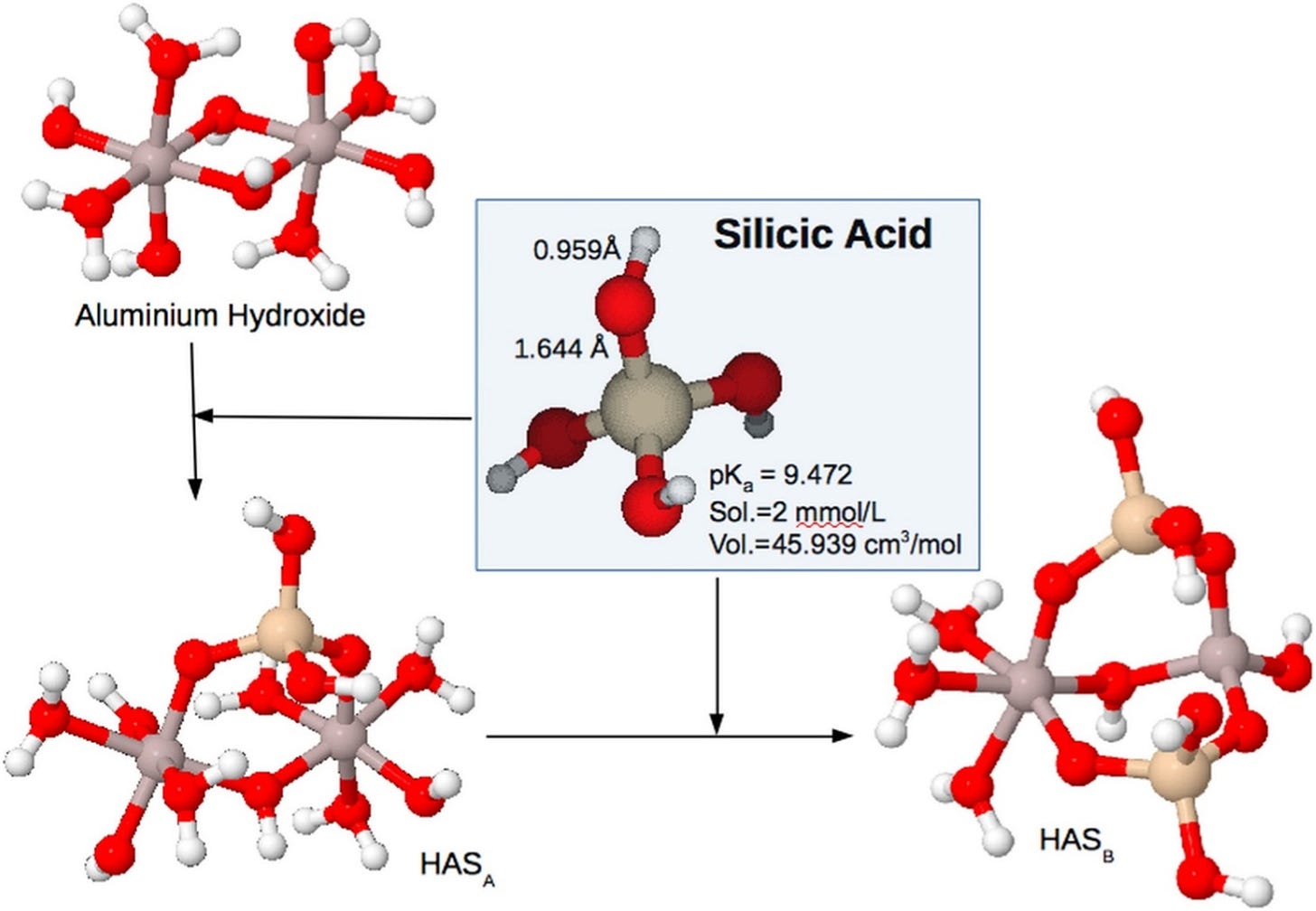
As a biologist I appreciated very early in my career that to understand aluminium and silicon I would need to understand a modicum of chemistry. I learned from many good chemists not least of which was a triumvirate of inorganic chemists based at Umeå in Sweden, Sjöberg, Öhman and Petterson. The latter delineated the chemistry whereby silicic acid was bound by a cage of molybdenum atoms, the reaction that is still used today to measure the concentration of silicic acid in water. Sjöberg and Öhman produced a series of published papers on the chemistry of aluminium and the chemistry of silicon. Fundamental research of immense importance and a steep learning curve for me at the time. I became friends with all three of these great chemists and published a paper on silicon with Sjöberg recently. These scientists along with others and notably by Father in Science, JD Birchall OBE FRS, helped me to understand how silicic acid protected against the toxicity of aluminium. This observation in fish was easily extrapolated to all life. Silicic acid prevented aluminium from participating in biochemical evolution. However, how might a small neutral molecule with little known chemistry bring about such a gargantuan achievement. This is the question that I set about answering almost forty years ago and I am extremely proud to say that we now know the answer. We have identified and delineated the unique inorganic chemistry that underlies the reaction of silicic acid with aluminium. We were not the first to show that there is a reaction between silicic acid and aluminium but we were the first to demonstrate and prove the mechanism of such a reaction. Silicic acid reacts, not with the free metal cation as previously supposed, but with the solid phase, aluminium hydroxide.
The products of the reaction are called hydroxyaluminosilicates as shown above.
I am sure that the delineation of this chemistry, the chemistry that underlies life on Earth as we know it today, is my greatest achievement in science.
Of course, as indicated in the silicic acid cycle earlier in this post, there are other important and fascinating reactions of silicic acid that define the bare necessities of silicon, such as I wrote about in my post on horsetail.
I promise to write more about these reactions in a future post.



Thank you for your work, and for sharing its importance with the world! It has been a honor and a blessing to know you!
I love reading your posts.