It was pleasing to observe that my recent post concerning the origin of life stimulated a varied discussion and not least upon Darwinian natural selection.
Some of the discussion suggested to me that many were struggling with the concept that natural selection acts as a force beyond that attributed to the evolution of biota. That is that it only acts upon the living. My thesis being that everything is subject to natural selection or survival of the fittest if you prefer. The latter, the fittest, is a product of an environment and there are myriad environments at play even, for example, within a single cell.
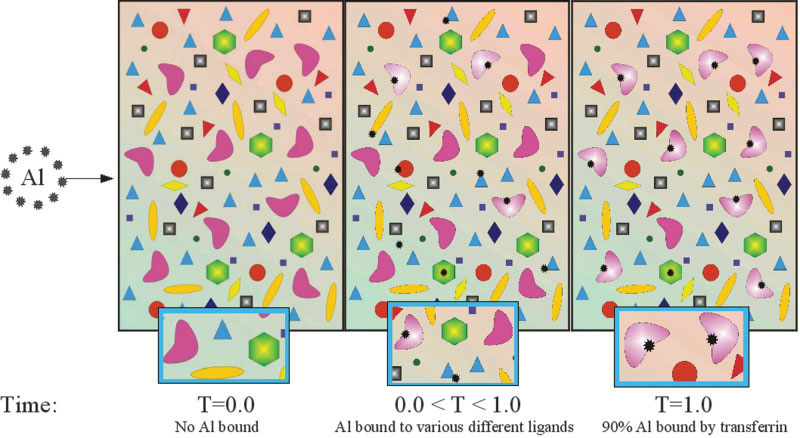
We can use the example of the blood-aluminium problem. To ascertain the distribution of aluminium in blood a sample is taken and various sophisticated fractionation techniques are applied to identify the location of aluminium. The result is invariably that over 90% of the aluminium is bound by the iron transport protein transferrin. So, the great majority of aluminium in blood is tightly bound in a complex with transferrin. However, urine is the major route of excretion of aluminium from the body. The blood is filtered by the kidney and aluminium is adventitiously removed from the blood to be excreted in urine. But the kidney filter, called the glomerulus, is much too small to allow the iron transport protein transferrin to pass through so if aluminium is bound to transferrin how can it be removed from the blood by the kidney. This is the blood aluminium problem and it is represented schematically in the figure below. See the original paper for details.
When a blood sample is taken and analysed the components in that sample are close to or at chemical equilibrium. This is represented as T=1.0 in the figure above. This raises the question as to where aluminium is located in blood prior to blood being removed from the body and thereafter being allowed to achieve chemical equilibrium. Assuming that you are alive and kicking then, as you read this, your blood will not approach chemical equilibrium. Just like the rest of your body and all that it consists of, your blood is in what might be termed as a dynamic equilibrium, far from the steady-state of chemical equilibrium. Without access to a special form of digital camera that can take a picture of your blood and its many components in situ we have to use computational chemistry to solve and prove the blood-aluminium problem. We have done this, at least my highly gifted PhD student James Beardmore did this. The figure below helps to explain how and why the kidney is able to filter aluminium from the blood. Again, see the original paper for details.
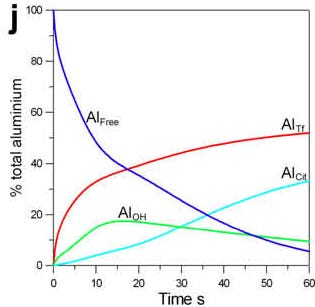
The highly innovative and entirely unique non-equilibrium computational chemistry developed by James shows the role of time in determining the distribution of aluminium in blood and, in this admittedly simple version of blood, demonstrates how ligands such as hydroxide (green line) and citrate (light blue line) compete with transferrin (red line) to bind free aluminium (dark blue line).
The identification of hydroxide as a significant early ligand for aluminium helps to explain why drinking a silicon-rich mineral water facilitates the excretion of aluminium in urine.
When silicic acid, the biologically available form of silicon in mineral waters, enters the blood it binds aluminium hydroxide to form small filterable hydroxyaluminosilicates. Silicic acid is not bound by free aluminium and so the observation that silicic acid increases the excretion of aluminium in urine proves that our model predicting a significant role for aluminium hydroxide in the blood-aluminium problem is correct. If only we had had the time and funding to include silicic acid in this computational model. Perhaps some other brave and adventurous soul will take this on in the future.
Natural selection taking place in the dynamic environment of blood dictates the distribution of aluminium between competitive ligands. We learn that it is not always the ligand with the highest affinity for aluminium, in this case transferrin, that has the most profound influence upon the biology of aluminium. If it were otherwise then aluminium would accumulate in the body to an even greater extent than is already the case. In this example, natural selection through total serendipity protects us from the toxicity of aluminium.



I thank you for the goodness and truth you bring into this world.
It is kind of miraculous that despite all of these toxic metals, we have a robust system that gives plenty of warnings that something is not right.
It's up to the culture whether this is acceptable or not.
These days, despite the corruption in medical science, we have the truth that can be explained to laymen.
It's real science that will destroy the scam that pretended to be science.