A Contribution to Science
Something for the text books of the future (assuming that there are any)
I am often asked about the science, my science, that I am most proud of. If I may be immodest, I actually find this a difficult question to answer since I am immensley proud of my scientific record and specifically the science detailed in over 200 peer-reviewed published papers. Good science is tough and getting good science that concerns a subject of controversy published by the establishment is even tougher. However, as a biologist (no Wikipedia, I am not an English chemist) it is probably my research demonstrating the reaction of aluminium with silicon to form hydroxyaluminosilicates that has brought the greatest personal satisfaction. It is also the subject that was born out of my PhD research and has remained pivotal to my science ever since.
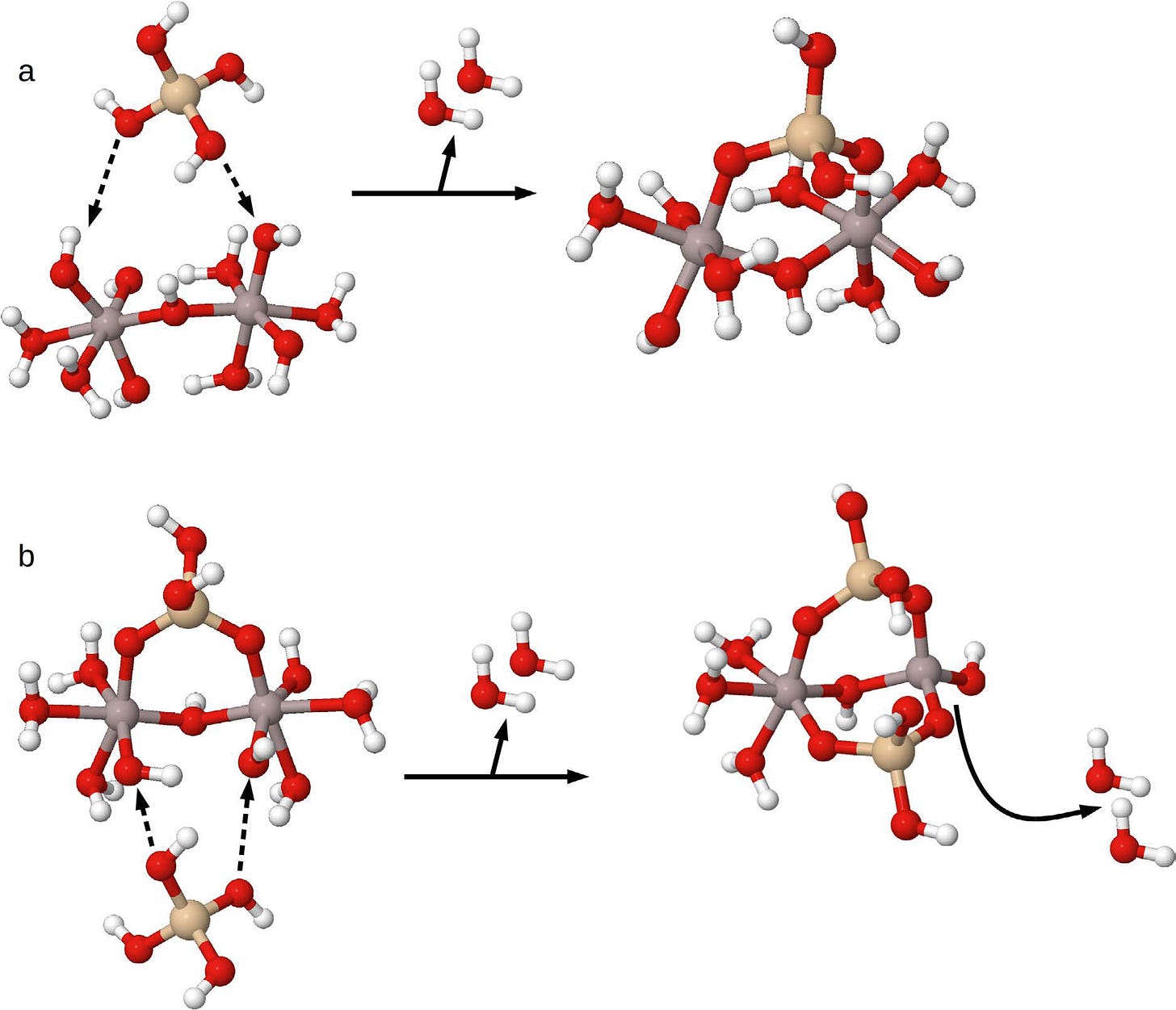
So what exactly am I referring to. Take a look at the chemical reactions depicted in the below image.
In ‘a’ the reaction of silicic acid (this is the form of silicon in a silicon-rich mineral water) with a dimer (two molecules) of aluminium hydroxide (the insoluble form of aluminium in water) forms hydroxyaluminosilicate A (HASA). You will notice that two molecules of water are lost in this reaction. HASA has a ratio of silicon to aluminium of 0.5, one silicic acid molecule bridging across two molecules of aluminium hydroxide.
In ‘b’ the reaction of a further molecule of silicic acid with HASA forms hydroxyaluminosilicate B (HASB). The second molecule of silicic acid bridges across hydroxyl (OH) groups on the opposite side to the first silicic acid molecule, again notice that this ‘bridging’ results in two molecules of water being expelled. HASB has a ratio of silicon to aluminium of 1.0, two silicic acid molecules bridging across two molecules of aluminium hydroxide.
Please believe me when I tell you that these reactions of neutral (uncharged) silicic acid with insoluble aluminium hydroxide and/or HASA are remarkable enough in the field of inorganic chemistry. However, even more incredible is what happens in the formation of HASB. You will notice that in aluminium hydroxide and in HASA each aluminium atom is surrounded by six groups, six bonds from each atom of aluminium. Look again at reaction ‘b’ and you will see that following the formation of HASB a further two molecules of water are expelled due to a rearrangement of the groups around one of the aluminium atoms. It is now surrounded by four bonds and not six.
It is this rearrangement of the bonds around just one of the aluminium atoms that makes HASB ultimately so stable. It is as if the aluminium atoms are now fully enclosed by silicic acid making them wholly inaccessible.
You have now learned the secret (until discovered by me) behind why silicon-rich mineral waters facilitate the removal of aluminium from the body. The higher content of silicic acid promotes the formation of HASB and these stable hydroxyaluminosilicates can then be filtered from the blood by the kidney.
Below is a summary figure describing these groundbreaking and unique reactions in inorganic chemistry. Take a copy of the figure and use it next time anyone asks you why you are a regular consumer of a silicon-rich mineral water.
The discovery of new chemistry is always exciting and even more so for a mere biologist. The discovery of the unique inorganic chemistry that explains how Nature kept aluminium out of living things for almost all of biochemical evolution is mind blowing. It certainly makes me proud and it should populate inorganic chemistry texts for many years to come. It is the antidote to the Aluminium Age.




Brilliant work. Loved your interview with Doc Malik, one of the best I have heard. Nice to hear something true for a change. Huge thanks. The truth is becoming an endangered species...
A question for Dr. Aluminum: I thought I was doing well at reducing my aluminum intake until someone pointed out that certain forms of salt–forms one would purchase and ingest–have been found to contain aluminum. Supposedly they were tested at an “EPA lab.” One producer of a brand found to have a lot of aluminum, 229 PPM, said the aluminum was in the form of “aluminosilicate.” The question is, if one consumes aluminum as aluminosilicate can it be converted into reactive aluminum say, by stomach acid or some other means? Can salt with aluminosilicate be safely eaten? I hope this makes sense and I hope I don’t have to throw away a bunch of salt.