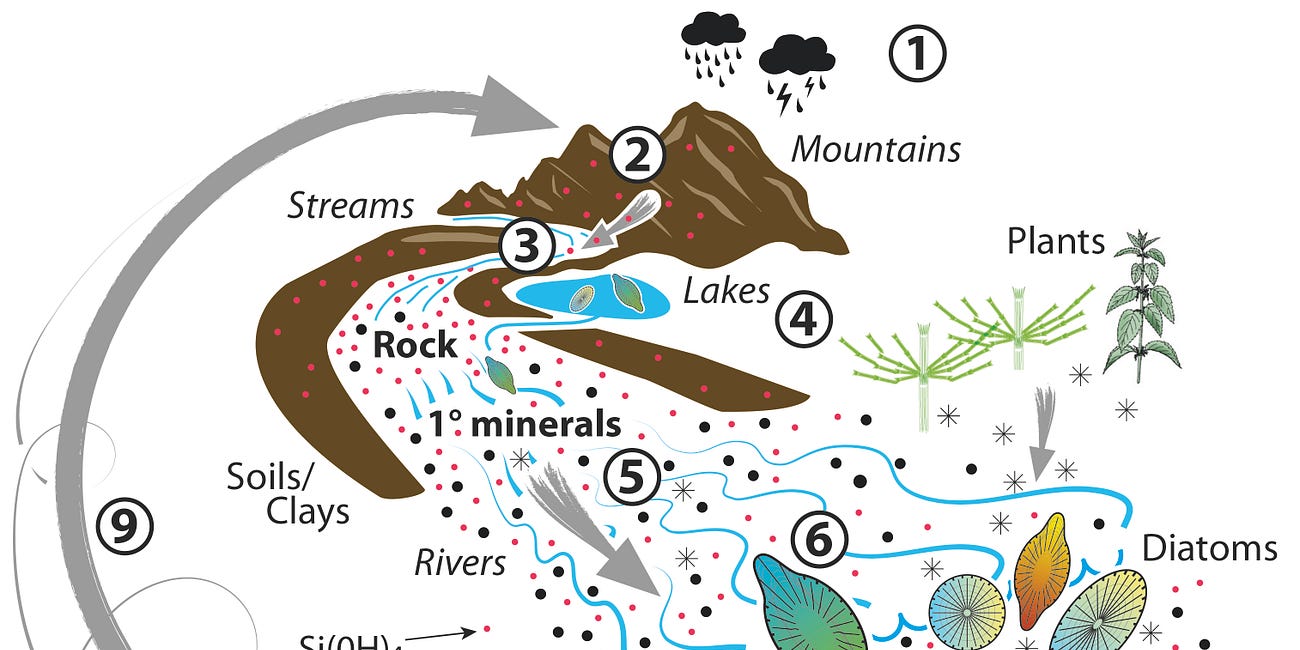
The Silicic Acid Cycle
Earth's thermostat
Recently I promised to write more about the bare necessities of silicon.
Silicon, A Bare Necessity of Life
The story of silicon in life is essentially the story of silicic acid. The Earth’s crust is composed primarily of three elements, oxygen, silicon and aluminium. Perhaps the most prominent examples of the Earth’s crust are mountains. In their upper reaches the Earth’s crust is directly exposed to the environment, to the elements. Rainfall dissolves mount…
Herein I am going to make a case for the silicic acid cycle as Earth’s thermostat. I am going to identify the major driving force behind so-called climate change.
Silicic acid plays a primary role in mineral weathering. As the significant soluble product of carbon dioxide driven dissolution of aluminosilicate rocks (mountains), silicic acid influences the kinetics or rate of mineral weathering. Through equilibrium dynamics, the concentration of silicic acid in any given environment is potentially a brake on mineral weathering.
The advent of biological silicification (see for example my post on horsetail)
Horsetail
Whenever I am asked to talk or write about the role of silicon-rich mineral waters in facilitating the excretion of aluminium from the body there follows the inevitable comment about how someone has been using some form of silicon supplement for years, ostensibly for the same reason. A common example of such is horsetail, often taken as a tea. Either in…
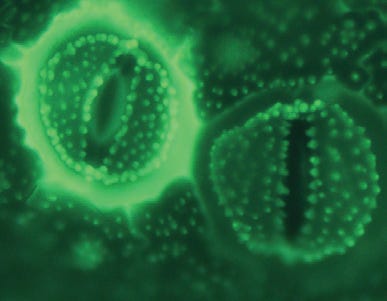
and primarily silicification by diatoms had (and has) the effect of lowering the concentration of silicic acid in the natural environment and releasing the brake on mineral weathering. Biological silicification (of which more to come in future posts) is a mechanism by which silicic acid harvested from environments in which it is significantly under-saturated is concentrated and subsequently deposited as amorphous biogenic silica. The advent of biological silicification in the evolution of life on Earth, through the removal of silicic acid from solution, acted as an accelerant of carbon dioxide fuelled mineral weathering and in consequence lowered atmospheric levels of carbon dioxide and contributed significantly to global cooling. The biological availability of silicon as silicic acid is acting as a cooling system for Earth so why is contemporary Earth warming.
An often-overlooked consequence of the burning of fossil fuels is a shift in the major acidifying anion in both wet (rain) and dry deposition. In many regions of the planet, the pH of rainfall is no longer governed by carbonic acid (pH 5.2-5.5), but by much stronger mineral acids and primarily sulphates (acid rain, pH 4.0-4.5). In these regions, there has been a switch in the major acidifying anion in rainwater brought about by release of sulphur and nitrous oxides in anthropogenic (coal, oil and gas) emissions. The consequences for mineral weathering and silicic acid as a global coolant are primarily two-fold.
The first is that sulphuric and not carbonic acid is the primary reactant in aluminosilicate weathering. Consequently, less atmospheric carbon dioxide is drawn down directly through mineral weathering.
The second is that the products of mineral weathering, where sulphate is the major anion, are different to those where bicarbonate is dominant (See equations 1&2 below.). This shift in pattern of mineral weathering is sometimes evident in regions heavily impacted by acid deposition as changes in clay mineral profiles. A further possible consequence of such a change in acid fuelled mineral weathering is a two-fold reduction in the stoichiometric release of silicic acid (See equations 1&2 below.).
The weathering of potassium feldspar (rock) where carbonic acid is the principal weathering acid in rainfall has a relatively slow reaction rate;
10–15.5 μmol m−2 s−1
2KAlSi3O8 + 2CO2 + 11H2O→Al2Si2O5(OH)4 + 2 K+ + 2HCO3− + 4Si(OH)4
K‐Feldspar Kaolinite Silicic Acid (1)
The weathering of potassium feldspar where sulphuric acid is the principal weathering acid in rainfall has a relatively fast reaction rate;
10–14.6 μmol m−2 s−1
2KAlSi3O8 + H2SO4 + 4H2O→Al2Si4O10(OH)2 + 2K+ + SO42− + 2Si(OH)4
K‐Feldspar Montmorillonite Silicic Acid (2)
Carbon dioxide fuelled aluminosilicate mineral dissolution acts as a slow-release system for silicic acid, while sulphuric acid fuelled dissolution is more rapid, but less productive per mole of aluminosilicate mineral.
I have argued that the industrial revolution and the concomitant acidification of the atmosphere primarily through burning fossil fuels resulted in an accelerated diminution of biologically available silicic acid. One possible consequence of such would be a fall in primary productivity, predominantly attributable to diatom populations in the oceans, and a concomitant reduction in the drawdown of carbon dioxide from the atmosphere.
Is this one of the (main) reasons why the Earth is warming?
If you would like to read more about this and access all the relevant references to my musings then please see my recent paper. Unfortunately it is not open access so if you cannot access the full paper online then do feel free to email me for a copy.
Finally, a friendly reminder that my posts are available free to all though anyone wishing to become a paid subscriber is helping me to maintain our website and through this and this substack the profile of aluminium and silicon in life.


"and in consequence lowered atmospheric levels of carbon dioxide and contributed significantly to global cooling"
I'm coming across quite a few mentions that the current level of CO2 in the atmosphere is 0.04%, towards the lower threshold of the level necessary for plant life to thrive. Also that CO2 is nowhere near as significant a contributor to global warming as water vapour, and that rises in CO2 levels follow global temperature changes by a lag of 800 years. There are so many pollutants out there (and in our bodies!) which should be reduced or banned, but it seems that plant-friendly CO2 and nitrogen compounds are being aggressively campaigned against - if successful, this would result in seriously stifling the well-being of human populations. Dr Patrick Moore, founding member of Greenpeace, spoke clearly about these topics recently on UK Column.
Have you seen this brilliant work up by @ethicalskeptic ? https://theethicalskeptic.com/2020/02/16/the-climate-change-alternative-we-ignore-to-our-peril/